Morforex
In today's world, Morforex has become a topic of utmost importance and relevance. Whether in the personal, professional, political or social sphere, Morforex has gained great relevance and has generated a wide debate among experts and society in general. The importance of Morforex lies in its direct impact on different aspects of daily life, as well as its influence on the development and evolution of different areas of knowledge and culture. This is why it is essential to analyze and understand in depth the importance and impact that Morforex has on our current reality, as well as to anticipate possible future scenarios that may arise as a result of its presence in various areas.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H24N2O |
| Molar mass | 248.370 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
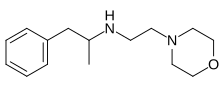
Morforex (INN; Bo 637), also referable to as N-morpholinoethylamphetamine, is an anorectic which was never marketed.[1][2]
It produces amphetamine as an active metabolite.[3]
Synthesis

Amphetamine is reacted with N-Chloroethylmorpholine in the presence of IPA solvent.
See also
References
- ^ Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1377–. ISBN 978-0-412-46630-4.
- ^ World Health Organization (2000). International Nonproprietary Names (INN) for Pharmaceutical Substances. World Health Organization. ISBN 978-0-11-986227-0.
- ^ Goodwin BL (10 November 2004). Handbook of Biotransformations of Aromatic Compounds. CRC Press. pp. 13–. ISBN 978-0-203-64196-5.
- ^ FR 2199M, "N-morpholino-1 n-phénylisopropylamino-2 éthane ", published 1963-12-09, assigned to Brevets Pharmaceutiques et Cosmetologiques
| Adamantanes | |
|---|---|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |