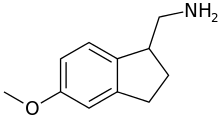
1-Aminomethyl-5-methoxyindane
In this article we will explore all aspects related to 1-Aminomethyl-5-methoxyindane, from its origin to its impact on today's society. We will analyze how 1-Aminomethyl-5-methoxyindane has influenced different areas, from culture to economics, including politics and technology. Additionally, we will examine the role of 1-Aminomethyl-5-methoxyindane in people's daily lives and how it has evolved over time. Through this comprehensive analysis, we aim to offer a complete and in-depth view of 1-Aminomethyl-5-methoxyindane, with the aim of providing a comprehensive understanding of its importance and relevance today.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15NO |
| Molar mass | 177.247 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
1-Aminomethyl-5-methoxyindane (AMMI), is a drug developed by a team led by David E. Nichols at Purdue University, which acts as a selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with similar affinity to DFMDA.[1][2]
See also
References
- ^ Roman DL, Saldaña SN, Nichols DE, Carroll FI, Barker EL (February 2004). "Distinct molecular recognition of psychostimulants by human and Drosophila serotonin transporters". The Journal of Pharmacology and Experimental Therapeutics. 308 (2): 679–87. doi:10.1124/jpet.103.057836. PMID 14593087. S2CID 6439942.
- ^ Walline CC, Nichols DE, Carroll FI, Barker EL (June 2008). "Comparative molecular field analysis using selectivity fields reveals residues in the third transmembrane helix of the serotonin transporter associated with substrate and antagonist recognition". The Journal of Pharmacology and Experimental Therapeutics. 325 (3): 791–800. doi:10.1124/jpet.108.136200. PMC 2637348. PMID 18354055.